Abstract
We reported the discovery of YF2, a EP300 (p300) and CREBBP (CBP) HAT activator which demonstrates selective cytotoxicity in HAT-mutated DLBCL cell lines and induces histone acetylation in vitro and in vivo. Recently, we have focused on the role of HAT induction in regulating immune response in DLBCLs. CBP has been shown to function as an enhancer that regulates GC B-cell fate by controlling antigen presentation and terminal differentiation. HAT mutations are linked to reduced MHC-II HLA transcripts due to decreased acetylation at the promoter regions which leads to decreased GC B:T cell entanglement and derangements in the GC reaction.
Numerous studies have reported the effect of epigenetic drugs on antigen presentation. This response is caused by disruption of the BLC6/SMRT/HDAC3 repressor complex which co-occupies enhancers in the MHC Class II loci. Previously, we demonstrated YF2 induced p300 mediated acetylation of BCL6; abrogating its activity. More recently, we demonstrated through RNA-seq that prolonged treatment of YF2 upregulated the expression of several MHC Class I-II genes resulting in the upregulation of the immune regulation signaling pathway. Further, treatment with YF2 up-regulated MHC-I and -II expression in a dose dependent manner.
In addition to regulating immune response, BCL6 is a master regulator of GC formation. We hypothesize that induction of HATs will modulate immune response in splenic and circulating B and T-cells. Immunized BALB/c mice were treated with saline or 50mg/kg YF2 for 10 days. Spleen and blood samples were analyzed by flow cytometry. We observed a significant reduction in splenic weight, B-cell frequency, and GCs formation in YF2 treated mice. YF2 treatment increased the frequency of mature and transitional B-cells in the spleen. Further, YF2 treatment reduced light zone and increased dark zone cells in the spleen. YF2 treatment also increased the frequency of plasma cells and plasmablast. MHC-I and -II expression was significantly upregulated in YF2 treated mice. YF2 exposure resulted in an increase of PD-L1hi B-cells. Lastly, circulating B-cells showed significant increase in MHC-II expression following YF2 treatment.
In splenic T-cells, we observed a reduction in CD4+ helper T-cells and an increase in CD8+ cytotoxic T-cells in YF2 treated mice. YF2 caused a reduction in naïve CD4+ cells and an increase in the frequency of central and effector memory cells Lastly, we examined the frequency of inhibitory molecules PD1, TIGIT, CD226, Lag3, and VISTA. PD-1, TIGIT, and CD226 were upregulated with YF2 treatment in CD4+ cells and CD226 was upregulated in CD8+ cells. Together, these results demonstrate YF2 is able to modulate immune response in B and T-cells.
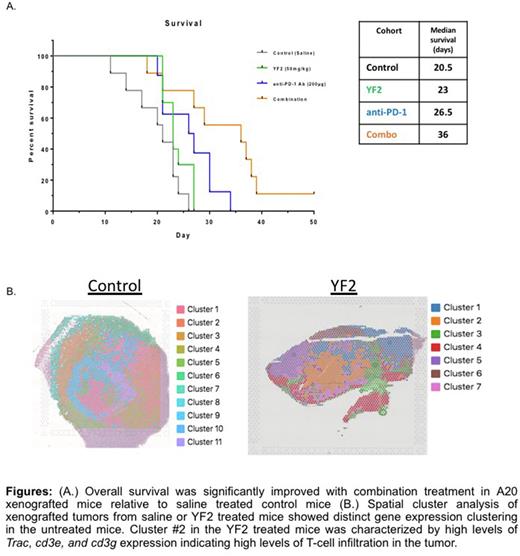
DLBCLs are known to have limited sensitivity to checkpoint blockade with PD-1 and PD-L1 inhibitors due to low PD-L1 and PD-1 expression which is in part driven by HAT mutations and overexpression of BCL6; which regulates PD-L1. We hypothesize that combination treatment of YF2 and PD-1 inhibitor in the immunocompetent syngeneic A20 mouse tumor xenograft model will result in decreased tumorigenicity and improved survival in mice. Xenografted and immunized BALBc mice were treated with saline, YF2, PD-1 inhibitor, or a combination of YF2 and PD-1 inhibitor for 28 days. We observed, a significant reduction in tumor volume in both the PD-1 and combination treated groups. Kaplan-Meier analysis showed an increase in median survival for all treatment with the combination treatment resulting in the greatest increase in median survival vs. control (Fig A). Spatial analysis was performed using the 10x Visium platform and sc-RNA-seq to examine the tumor microenvironment in control and YF2 treated mouse tumor samples (Fig B). We observed a more aggressive phenotype in the untreated tumors as well as an increase in the number of distinct clusters indicating a more heterogenous tumor population.
Lastly, PBMCs from patient samples were isolated and treated with YF2. Compared to healthy donor patients, YF2 induced cytotoxicity across various lymphoma subtype. Normal PBMCs were not affected by treatment. Based on the success of YF2 in the preclinical setting, the use of this first-in-class drug for treatment of lymphoma was approved to proceed in clinical studies in patients with relapsed/refractory lymphoma. Patient samples will be collected and analyzed for immune response.
Disclosures
Amengual:AstraZeneca: Consultancy; Appia Pharmaceuticals: Research Funding; Daiichi Sankyo: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal